Treatment of alpha-1
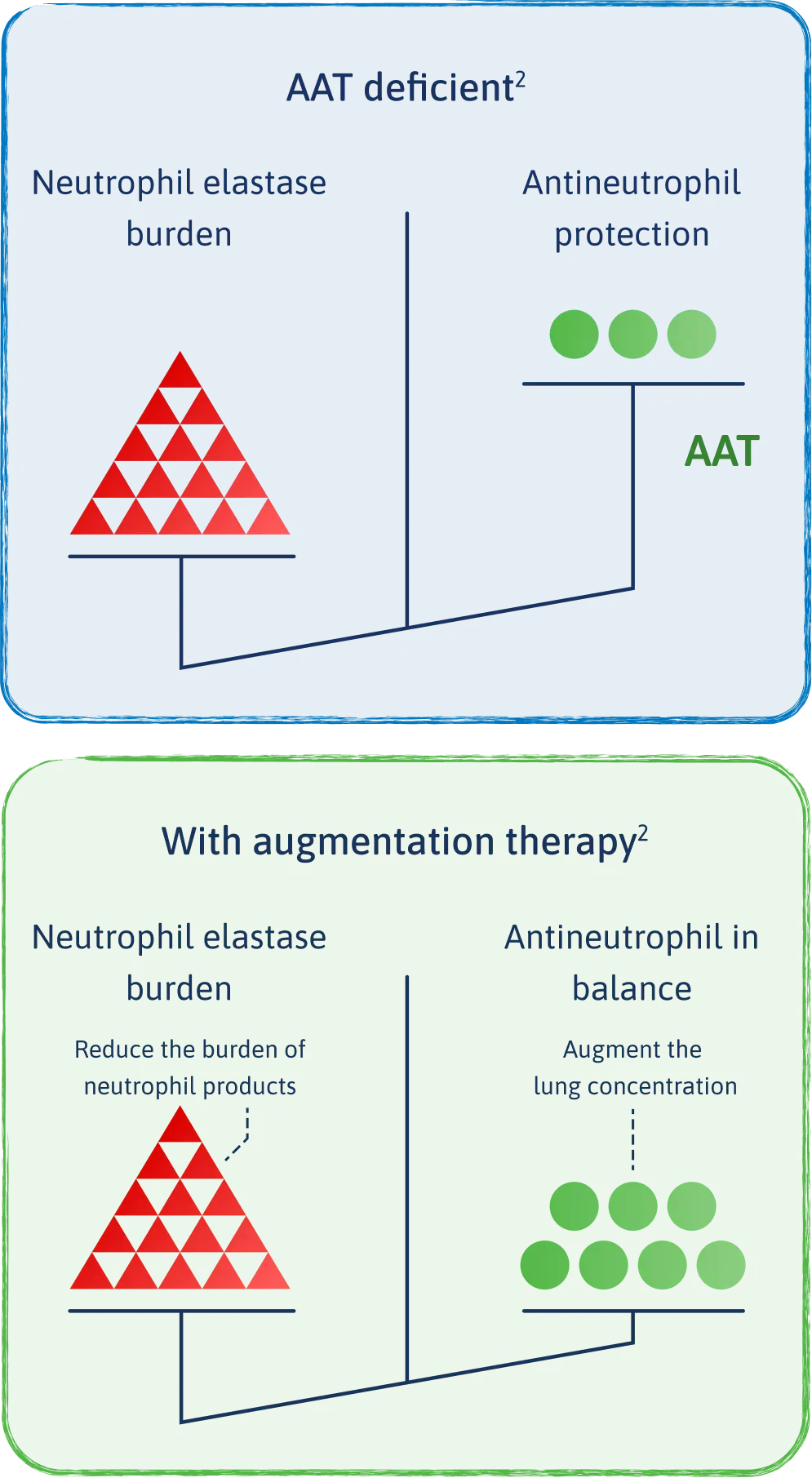
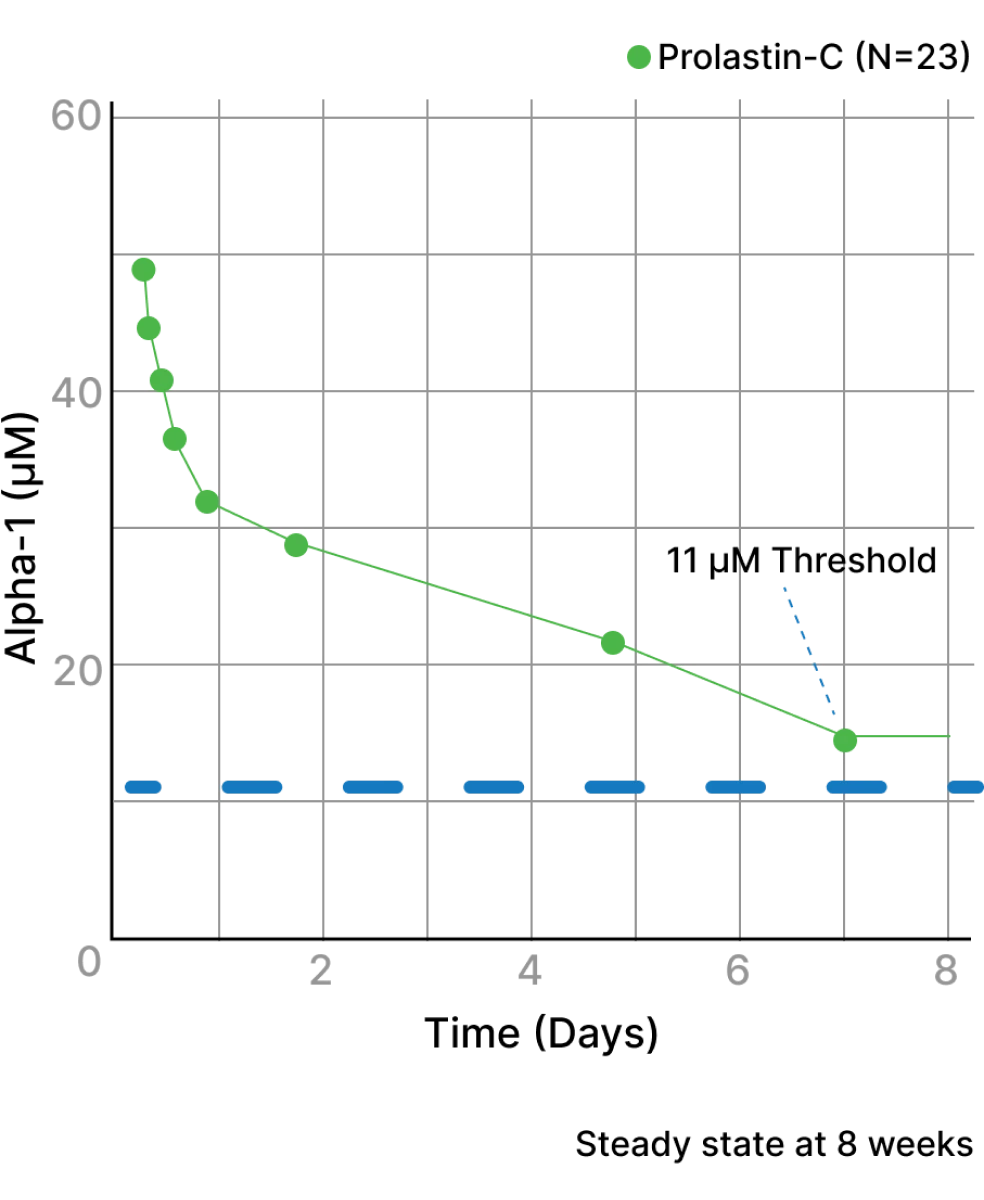
Augmentation therapy is proven to effectively raise alpha1 antitrypsin (AAT) levels in patients with alpha-1 that help keep neutrophil elastase in check.2
- Augmentation therapy is the only treatment specifically approved for adults with emphysema due to severe deficiency of alpha-1 protein.1
- The intended theoretical goal is to correct the imbalance between neutrophil elastase and AAT.1