How to administer PROLASTIN-C LIQUID
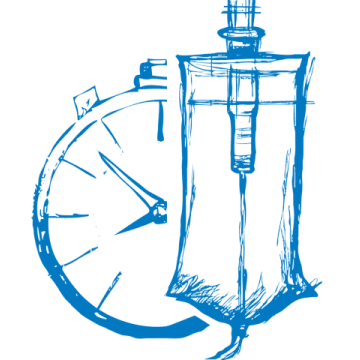
PROLASTIN‑C LIQUID is administered as a once-weekly intravenous (IV) infusion at a dose of 60 mg/kg body weight. The product is ready to use—no reconstitution required.1
PROLASTIN-C LIQUID should be given intravenously at a rate of 0.08 mL/kg/min as determined by the response and comfort of the patient.1

Dosing regimen
For intravenous use only
Warning and precautions
- Hypersensitivity reactions, including anaphylaxis, may occur.
- Monitor vital signs and observe the patient closely throughout the infusion.
- Early signs and symptoms may include: pruritus (itching), generalized urticaria (hives), flushing, swollen lips, tongue, or uvula.
NOTE: If hypersensitivity symptoms occur, promptly stop PROLASTIN-C LIQUID infusion and begin appropriate therapy.

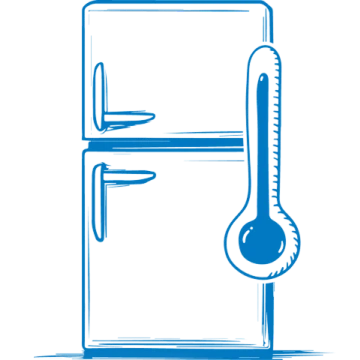
Storage and handling
Follow proper storage and handling to maintain product integrity and ensure safe administration.
PROLASTIN-C LIQUID is shipped refrigerated but allows for short-term room temperature storage.
Do not freeze.1

Enrollment form
Fill out the enrollment form to get your patients started on PROLASTIN-C LIQUID.
Important Safety Information
PROLASTIN®-C LIQUID is an alpha1-proteinase inhibitor (human) (alpha1-PI) indicated for chronic augmentation and maintenance therapy in adults with clinical evidence of emphysema due to severe hereditary deficiency of alpha1-PI (alpha1-antitrypsin deficiency).
Limitations of Use
- The effect of augmentation therapy with any alpha1-PI, including PROLASTIN-C LIQUID, on pulmonary exacerbations and on the progression of emphysema in alpha1-PI deficiency has not been conclusively demonstrated in randomized, controlled clinical trials.
- Clinical data demonstrating the long-term effects of chronic augmentation or maintenance therapy with PROLASTIN-C LIQUID are not available.
- PROLASTIN-C LIQUID is not indicated as therapy for lung disease in patients in whom severe alpha1-PI deficiency has not been established.
PROLASTIN-C LIQUID is contraindicated in immunoglobulin A (IgA)-deficient patients with antibodies against IgA or patients with a history of anaphylaxis or other severe systemic reaction to alpha1-PI products.
Hypersensitivity reactions, including anaphylaxis, may occur. Monitor vital signs and observe the patient carefully throughout the infusion. If hypersensitivity symptoms occur, promptly stop PROLASTIN-C LIQUID infusion and begin appropriate therapy.
Because PROLASTIN-C LIQUID is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens.
The most common adverse reactions during PROLASTIN-C LIQUID clinical trials in >5% of subjects were diarrhea and fatigue, each of which occurred in 2 subjects (6%).
Please see full Prescribing Information for PROLASTIN-C LIQUID.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References
- PROLASTIN®-C LIQUID (alpha1-proteinase inhibitor [human]) Prescribing Information. Grifols.
- Data on file, Total Prolastin Patients Treated 2015–2024, Grifols.