Supporting patients leads to better outcomes
Through PROLASTIN DIRECT, patients are automatically enrolled in the AlphaNet Alpha-1 Disease Management and Prevention Program (ADMAPP), which provides one-on-one support from a trained patient with alpha-1 who fully understands their condition.2
Health Outcomes Study Results (N=878)3†
Significant improvements in compliance with therapy‡
- Participants were members of AlphaNet, a not-for-profit health management company founded by people with alpha-1 to provide comprehensive care solely for people with alpha-13
- 97% of ADMAPP study participants were taking PROLASTIN-C LIQUID3
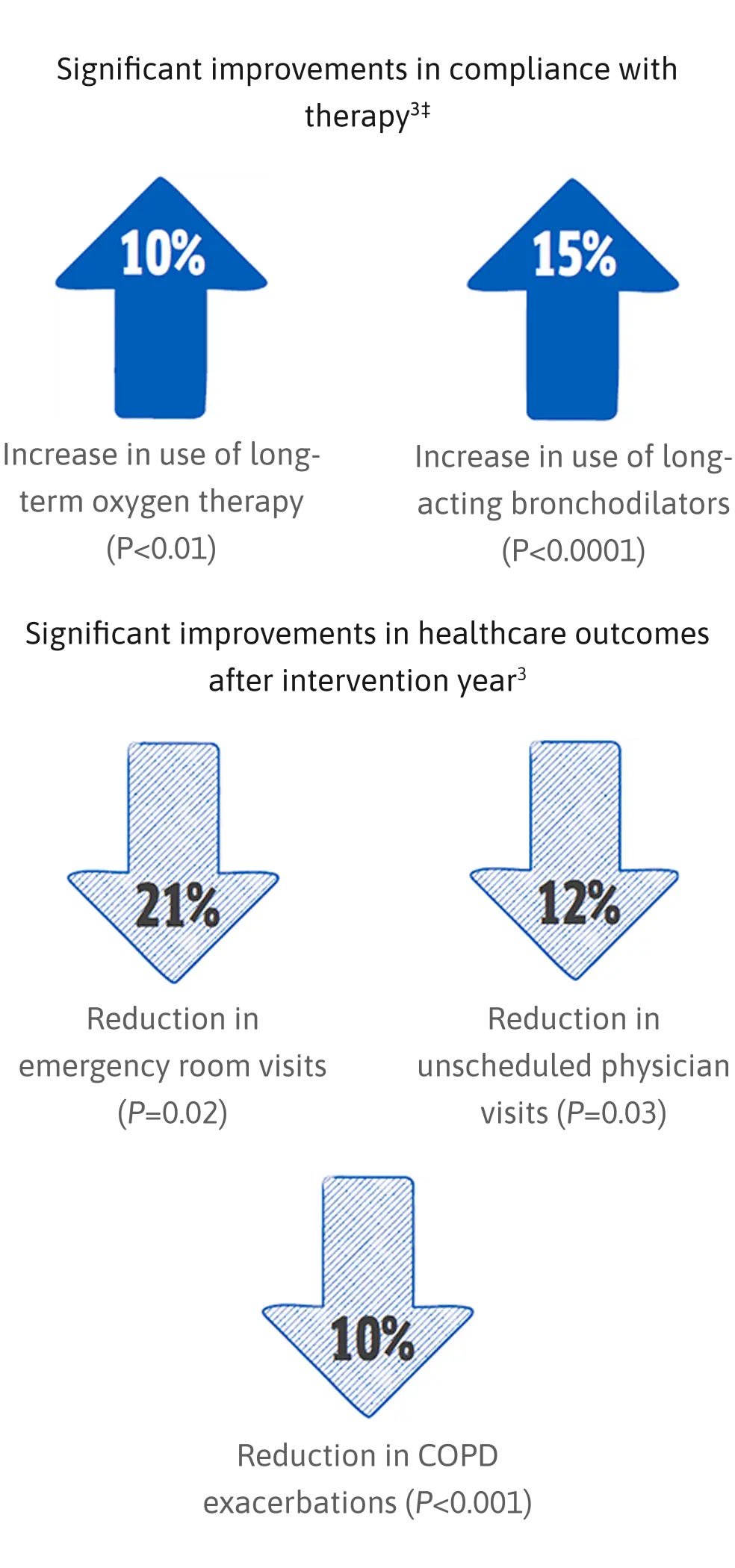
† 2-year study comparing outcomes in a 12-month observation period with augmentation therapy alone followed by a 12-month intervention period with augmentation therapy plus ADMAPP.3
‡ 10% increase in compliance with long-term oxygen therapy use, and 15% increase in use of long-acting bronchodilators, which means more optimal medication use.3