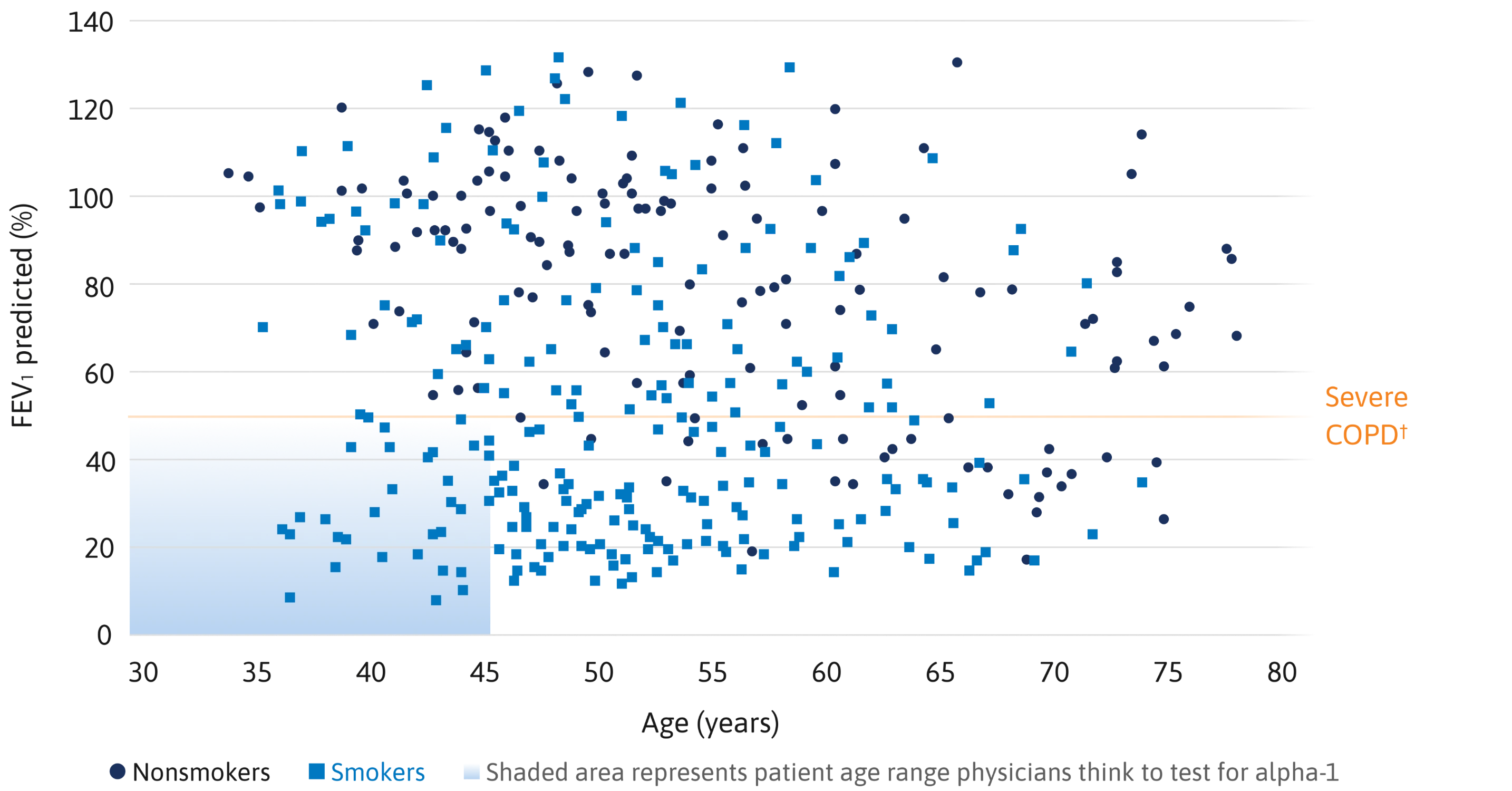
Survival analysis of individuals with severe alpha-1 (Pi ZZ) vs total US population8
A landmark study indicated that severe alpha-1 significantly shortened life span.8
Mean survival of the alpha-1 cohort (n=120) was 16% at 60 years of age, compared with 85% for the general population.8
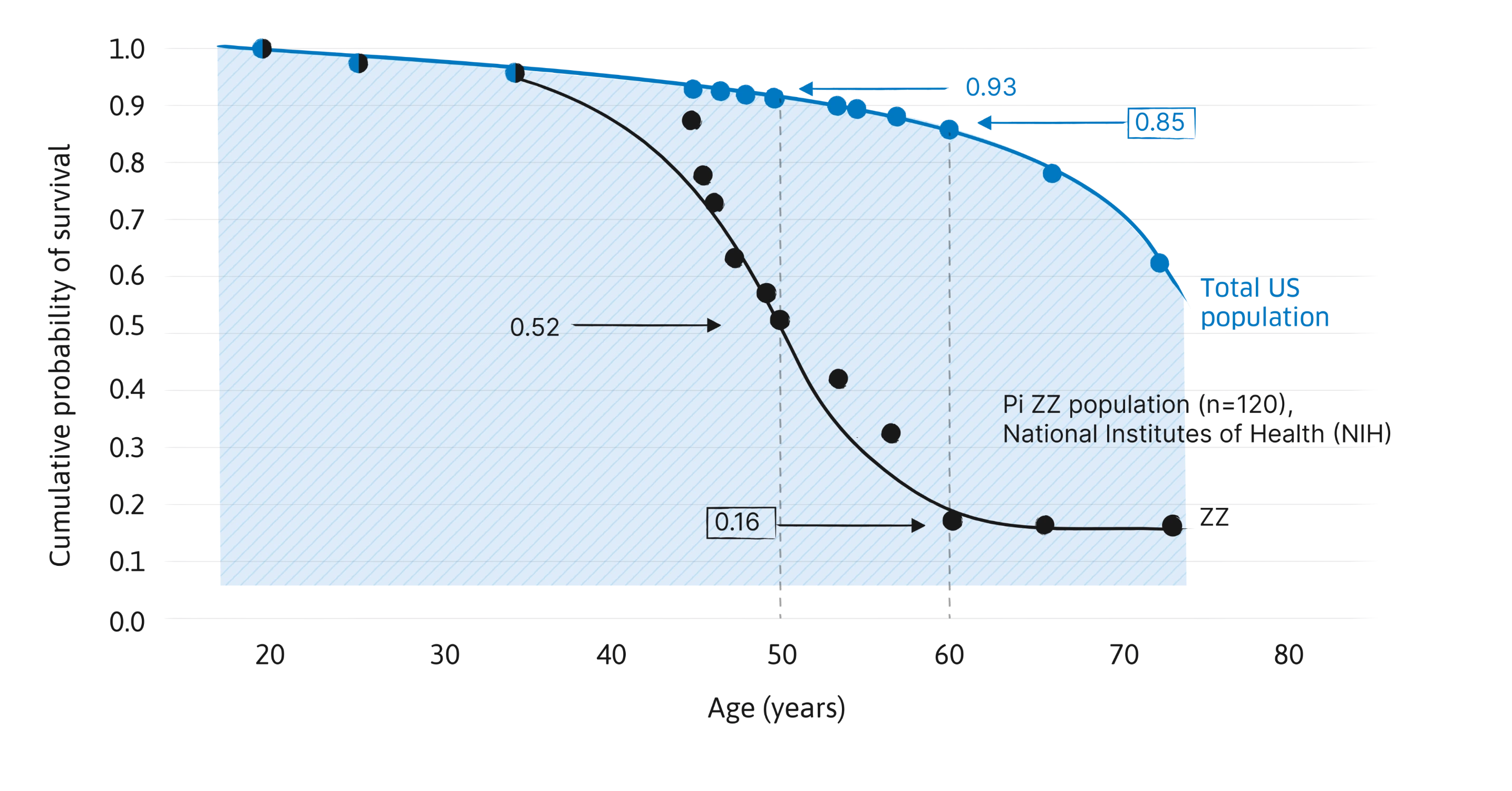
Used with permission of the American Thoracic Society. Copyright 2012, American Thoracic Society. Study Design, 1988: All 120 Pi ZZ subjects evaluated as inpatients in NIH Clinical Center. Various demographic features, clinical findings, and electrocardiogram evaluations were obtained from medical records. Blood and urine laboratory findings were obtained through computerized archives of the Clinical Center Laboratories. Chest radiographs and scintigraphy scans were evaluated de novo at the time of this analysis, with knowledge of the diagnosis but without knowledge of any of the other data. Lung function tests were obtained from the computerized files of the Pulmonary Branch. Mortality data were obtained at the time of this analysis. Alpha-1 antitrypsin protein phenotypes were determined by a combination of isoelectric focusing of serum, quantitation of AAT levels in serum, and family studies.8